Physical forces create chemical reactions
ME Online
April 4, 2007
Usually, a chemical reaction needs activators such as heat or light to help form or destroy chemical bonds. But now, scientists at the University discovered a new way to instigate and control the outcome of chemical reactions: mechanical force.
This discovery combines both chemistry and physics into the science of mechanochemistry, where applied physical forces can create chemical reactions and lead to a pre-determined outcome.
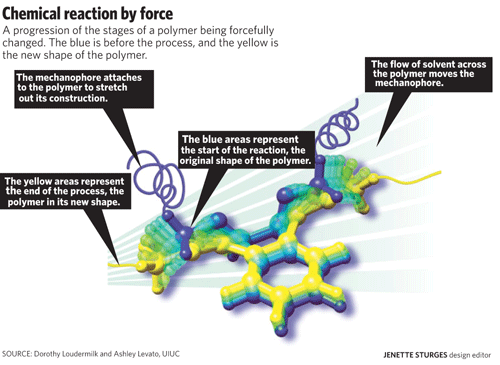
Professor Jeffrey Moore, professor of chemistry and one of the three professors heading the project, said the chemical reaction begins with a mechanophore. When this molecule is placed in the middle of a polymer chain, or a chain of molecules, it can be stretched and bent in different ways. In their experiment, Moore and his colleagues placed the polymer chain in water and then used ultrasound to create bubbles in the water. The bubbles would then collapse and create shockwaves that would pull the polymer chain, and essentially the mechanophore itself, manipulating the mechanophore’s shape.
“By applying force to the mechanophore, we could choose the end product of the reaction,” Moore said.
According to Autonomic Healing Research Web site, one of the possible applications of this research is the self-restoration of material. For example, if a helicopter blade was cracked, the force that created that crack could start a chemical reaction and create a substance in the crack that would both heal the damage and prevent further damage from occurring.
Get The Daily Illini in your inbox!
Moore also said another possible application allows for the identification of damaged materials. If a plane’s wing was damaged due to excessive use or age, the pressure from the damage could start a chemical reaction that would change the color of the damaged area, informing the pilot that the plane is not safe to fly.
This project took roughly five years to complete. Moore said it was a long and, at times, disheartening process.
“We worked for several years with no results,” Moore said. “But when it finally happened, you could imagine the excitement,”
Moore also said a large part of the success could be attributed to former grad student Chuck Hickenboth, who spent his entire graduate study on the project and was the only graduate student assigned to it.
“It was a roller coaster of a project, and by the third year, I was worried there wouldn’t be any results,” Hickenboth said. “But then things started clicking.”
Hickenboth did a majority of the data collection and analyzation during his time on the project.
He also designed and synthesized the molecules used in the experimentation process. Though he graduated in December, he wrote a majority of an article published in the March 22 edition of Nature, an international weekly journal of science. Moore said Hickenboth’s desire to see the project to its completion spurred the scientists forward in their work.
“I wanted to see something come out of it, and I didn’t want to throw it away,” Hickenboth said. “I didn’t want to leave it at ‘maybe.’ I didn’t like that answer.”
Moore and his colleagues will continue looking for other types of mechanophores. They hope to find more mechanophores and to make the potential applications possible.
“There’s a lot of interesting possibilities,” Moore said. “It’s kind of a big deal.”






