University team charged over new microbattery potential
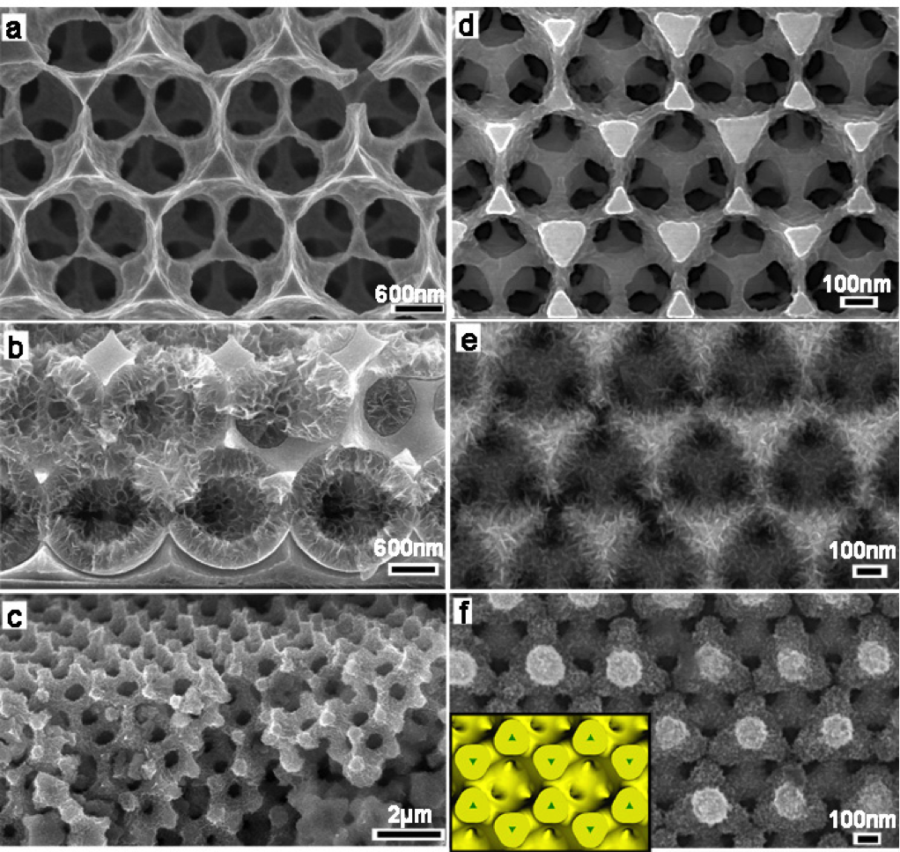
Images of the creation of the 3-D architecture, depicting the anode in images a–c and the cathode in images d–f.
December 3, 2014
In the not-so-distant future, students who have been waiting hours for a cellphone battery to recharge may only need to wait for mere minutes.
The departments of mechanical engineering and materials science and engineering are collaborating to create a lithium-ion microbattery that could charge up to 1,000 times faster than other technologies already on the market.
The research began about five years ago, and scientists are continuing to work on creating a battery that is more powerful than their small size suggests.
The basic process that takes place in a lithium-ion battery involves the oxidation of lithium, which then becomes a lithium ion and an electron. The lithium ion and electron begin moving from the anode to the cathode.
“The rate at which you can discharge or charge a battery depends on how fast the electron and lithium-ion can move,” said James Pikul, a graduate student in materials science and engineering and the first author of the publication on microbatteries. “The transport of lithium-ion is much slower than the transport of the electron. So the most power you can get out of the battery depends on how fast you can move that lithium-ion back and forth.”
Get The Daily Illini in your inbox!
Researchers are working to speed up the process in which the lithium and electron move from the anode to the cathode by creating a nano-architecture within the battery. The structure is nano-porous and resembles a sponge, with pores that are extremely small (500 nanometers, to be exact).
Another method of increasing speed is by creating a 3-D structure within the battery, which allows for more reactions to happen — and at a faster rate, said Jinyun Liu, a postdoctoral scholar in materials science and engineering.
“We make it 3-D because we want to achieve very high power density, and the reaction speed can be faster,” said Liu, whose work involves nano-structuring active materials for microbatteries.
The lithium and electron face many resistances as they travel through materials in the battery. The total resistance in a circuit is related to the distance they have to travel, and the area over which they have to travel, according to Pikul.
“Part of the design of the 3-D architecture is to make the distance smaller and to make it faster for these ions to travel through that distance,” Pikul said. “If you can make that distance smaller, you can drastically improve the time it takes to travel across it.”
Pikul said there are a couple of different directions being taken with this research. One of which is the microbattery thrust, which is the area Pikul is working on.
“The idea is that you can put this battery, which has a lot of power and energy, directly onto integrated circuits,” he said.
There is also work being done on macroscopic batteries, Pikul said. The idea for these batteries is to scale them up to a larger size so they can be used for things like cars or cellphones.
“One of the biggest resistances for electric vehicles is that it takes several hours to charge your car,” Pikul said. “So if you could increase the rate at which you can charge your car so that you only charge a car in five minutes, it becomes a lot more reasonable for people to adapt electric vehicles.”
Pikul said that initially, regarding the use of these batteries in cellphones, they would be a component of the phone, not a full replacement of a current phone battery.
“But eventually, you could get there,” he said.
Junjie Wang, a graduate student in materials science and engineering who is also working on the lithium-ion batteries, said the team is working to discover and use materials that will improve the functionality of the battery as well.
“We are now trying to apply these structures to new kinds of materials that can have a higher capacity or higher power density,” he said.
While the researchers are working hard to commercialize the battery, a date as to when it will become available to the public is yet to be determined.
Abrar can be reached at [email protected].